ACROSS THE WORLD



About Us
Innovative solutions for in-vitro diagnostics
With our Gel Centrifugation kit and equipment developed in the field of immunohematology, Dia Pro has rapidly become one of the few companies to gain worldwide recognition in in-vitro diagnostics. In just 10 years, we've established ourselves as a leader in the industry.
Our commitment is to provide cutting-edge technology, ensuring accuracy and reliability in diagnostic processes. We continue to innovate and expand our product range to meet the evolving needs of the medical community.
Global Presence of Dia Pro
Our Global Success Story
Today, Dia Pro exports its advanced gel centrifugation kits and immunohematology equipment to more than 40 countries across Europe, Asia, the Middle East, Africa, and Latin America. Our rapid growth in the global in-vitro diagnostics market is a testament to the quality, reliability, and innovation behind our solutions. By building long-term partnerships and maintaining a strong distribution network, Dia Pro continues to support laboratories and healthcare institutions worldwide with trusted diagnostic technologies.

R&D
Research & Development (R&D)
Certified by the Republic of Türkiye Ministry of Industry and Technology, our R&D Center develops innovative solutions in the fields of pre- and post-transfusion testing and microbiological diagnostic kits. We continuously improve production processes while designing next-generation diagnostic technologies.
With an integrated biotechnology laboratory, we work on advanced projects involving recombinant blood group antigens and custom protein design. We have successfully completed several TÜBİTAK projects in the field of transfusion medicine, and are currently running one TÜBİTAK and one KOSGEB project focused on biotechnology and molecular diagnostics. In-house funded projects also continue to enrich our scientific portfolio.
- Innovative Diagnostics
- Research Excellence



products
Dia Pro Product Range
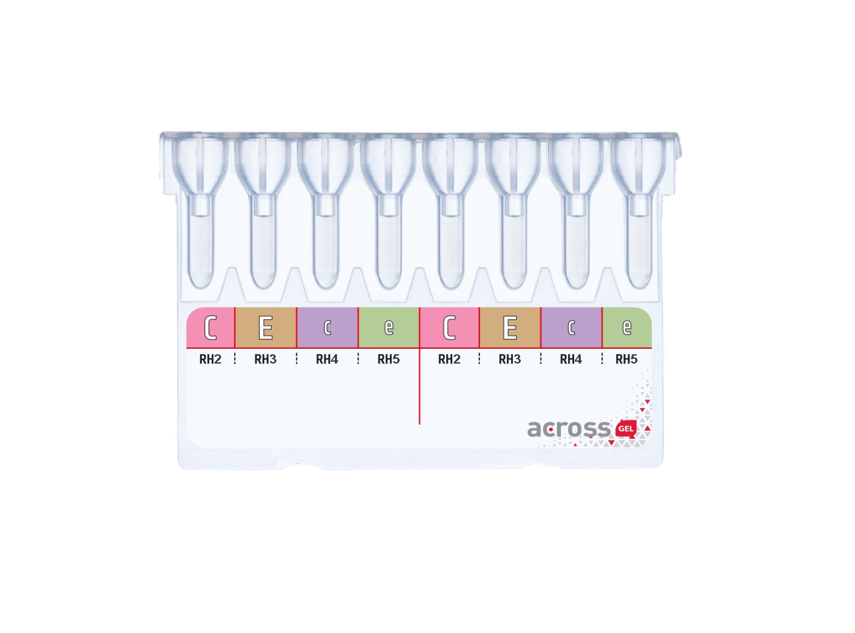
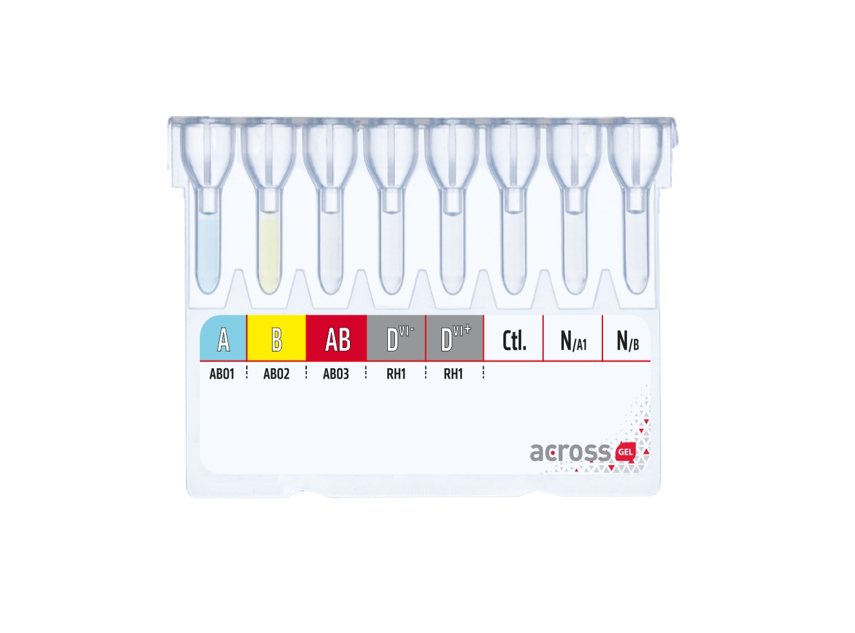
Dia Pro offers high-quality products for immunohematology, microbiology, serology, and in-vitro diagnostics. Our range includes gel centrifugation kits, diagnostic kits, sterile water products, and medical devices, meeting global healthcare needs with innovative solutions.